CLASSIFICATION OF PROTEINS ACCORDING TO THEIR STRUCTURE
 |
| Proteins |
The majority of proteins are compact, highly convoluted molecules with the position of each atom relative to the others determined with great precision. To describe the structure of a protein in an organism, it is necessary to specify the three-dimensional shape that the polypeptide chain assumes. Every protein has particular properties which are dictated by the number and the particular succession of amino acids in a molecule. There are basically three levels of structural organization in proteins.
- Primary Structure
- Secondary Structure
- Tertiary Structure
Some proteins also possess a fourth structure called Quaternary structure.
The sequence of the amino acids combined in a peptide chain is referred to as the primary structure. Peptide bond held together the primary structure which is made during the course of protein biosynthesis. F. Sanger was the first researcher who determined the arrangement of amino acids in a protein atom. Following ten years of cautious work, he finished up, that insulin is made of 51 amino acidsin two chains. One chain had 21 amino acids and the second had 30 amino acids in two alpha and two beta chains. Every alpha chain contains 141 amino acids, while every beta chain contains 146 amino acids.
 |
| Insulin Structure |
There are more than of 10,000 proteins in the human body which are made up of particular sequence of 20kinds of amino acids. This arrangement depends on the sequence of nucleotidesin the DNA. The sequence of amino acids in a protein particle is highly definite for its proper working. On the off chance that any amino acid is not in its typical place, the protein does not carry on its ordinary function. In this case, the sickle cell hemoglobin is the best example. Only one amino acid in every beta chain out of the 574 amino acids don't take up the ordinary place in the proteins (this specific amino acid is displaced by some other amino acid), and the hemoglobin fails to carry sufficient oxygen, thus prompting demise of the patient.
The secondary structure of protein is a regular coilingor zigzagging of polypeptide chains produced by hydrogen bonding between NH and C=O groups of amino acids near each other in the chains. They typically curl into a helix, or into some other consistent setup. One of the normal secondary structures is the a-helix. It has one winding arrangement of the basic polypeptide chain. The a-helix is an extremely uniform geometric structure with 3.6 amino acids in each one turn of the helix. The helical structure is maintained by the hydrogen bonds between amino acid atoms in consecutive turns of the winding. B-pleatedsheet is structured by folding over of the polypeptide.
 |
| B-pleated sheet |
Tertiary structure:
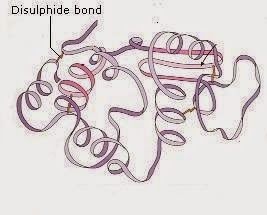
The three dimensional twisting and folding of the polypeptide chain results in the tertiary structure of proteins. Polypeptide chain twists and folds upon itself shaping a globular shape. The proteins' tertiary conformity is held by three kinds of bonds, i.e. Hydrogen, ionic and disulfide(-S-S-). In the aqueous environment, the steadiest tertiary conformity is that in which hydrophobic amino acids are covered inside while the hydrophilic amino acids are on the surface of the atom.
Quaternary structure:
In complex proteins, polypeptide tertiary sequences are coiled in multi-complex subunits. Quaternary proteins are made up of the arrangement of two or more tertiary chains and are held together by hydrophobic interactions, ionic and hydrogen bonds. Hemoglobin, the oxygen transport protein has such a structure.
Classification of proteins:
Due to the diversity and complexity in proteins’ structure, it is extremely hard to classify proteins in a single general category. So, as per their structure, proteins are categorized as follows:
Fibrous proteins:
They contain atoms having one or more polypeptide chains as fibrils. They are water insoluble and form tendons, muscle fibers, connective tissues and bone matrix. Secondary structure is of great importance in them. They are water insoluble, non-crystalline and elastic. They form tendons, muscle fibers, connective tissues and bone matrix. Myosin, silk fibers, fibrin, and keratin are examples.
Globular proteins:
Globular proteins are ellipsoidal and spherical because of numerous wrinkling of polypeptide chains. Tertiary structure is most essential in them. They are soluble in water and can be crystallized. They adjust themselves with changes in the physical environment. Cases are enzymes, hormones, antibiotics and hemoglobin.
Comparison between Fibrous and Globular Proteins:
| Fibrous Proteins | Globular Proteins | |
|---|---|---|
1. Fibrous Proteins are extended proteins and are insoluble in water. | 1. Globular Proteins are compact proteins and are soluble in water. | |
2. They have long thread like structures. | 2. They have folded ball like structures. | |
3. They have comparatively stronger intermolecular forces. | 3. They have weak intermolecular hydrogen bonding. | |
4. They have helical or sheet like structures. | 4. Globular have three dimensional shape. | |
5. They form structures of body or cells e.g. tendons, ligaments, hairs, etc. | 5. They perform metabolism, transport, immune protection, hormones, etc. |





.gif)
Comments 0
EmoticonEmoticon